ADEMPAS DELIVERS SIGNIFICANT IMPROVEMENTS ACROSS 4 CRITICAL PARAMETERS1

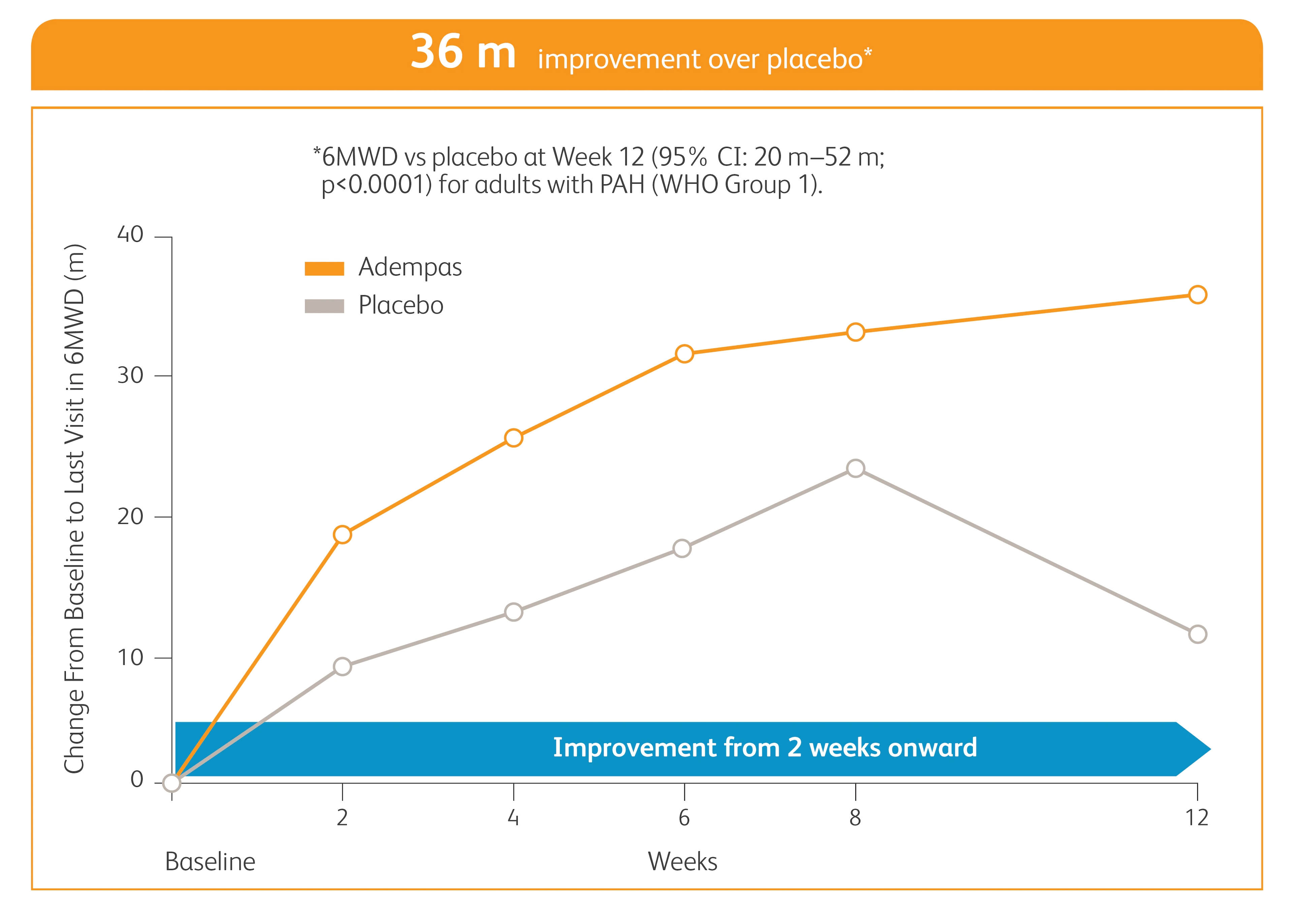
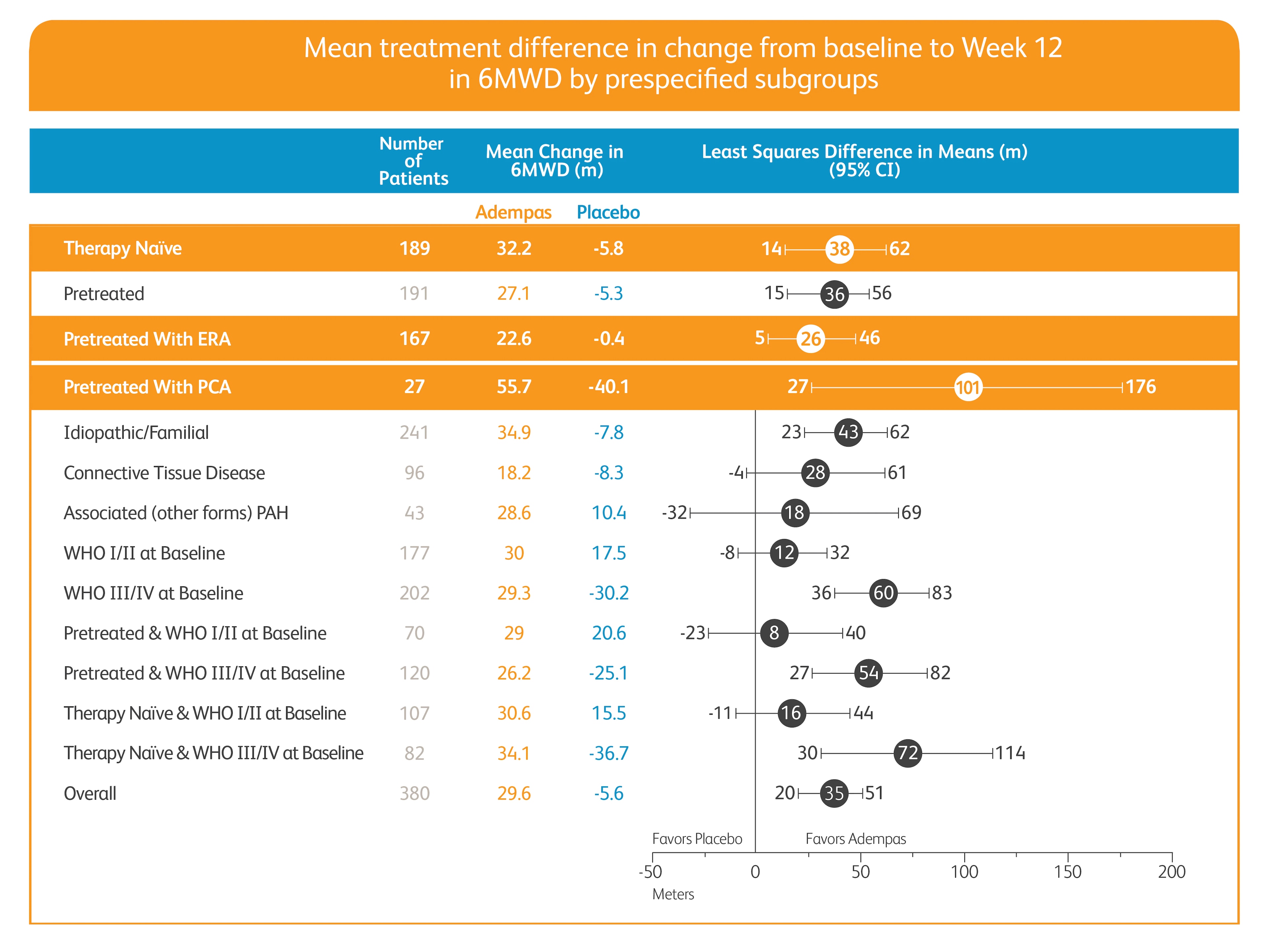
Improvement in 6MWD
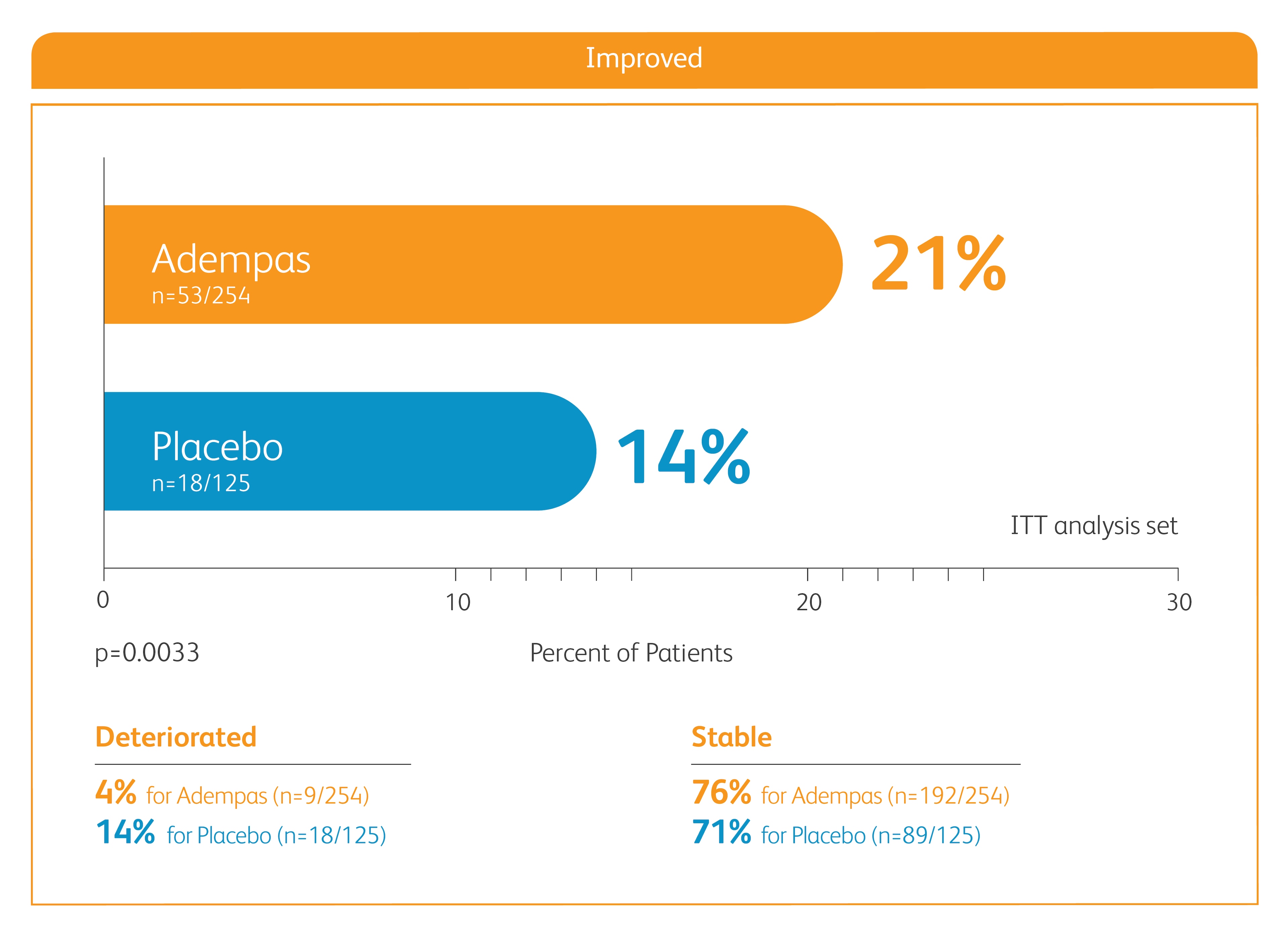
Improvement in WHO FC

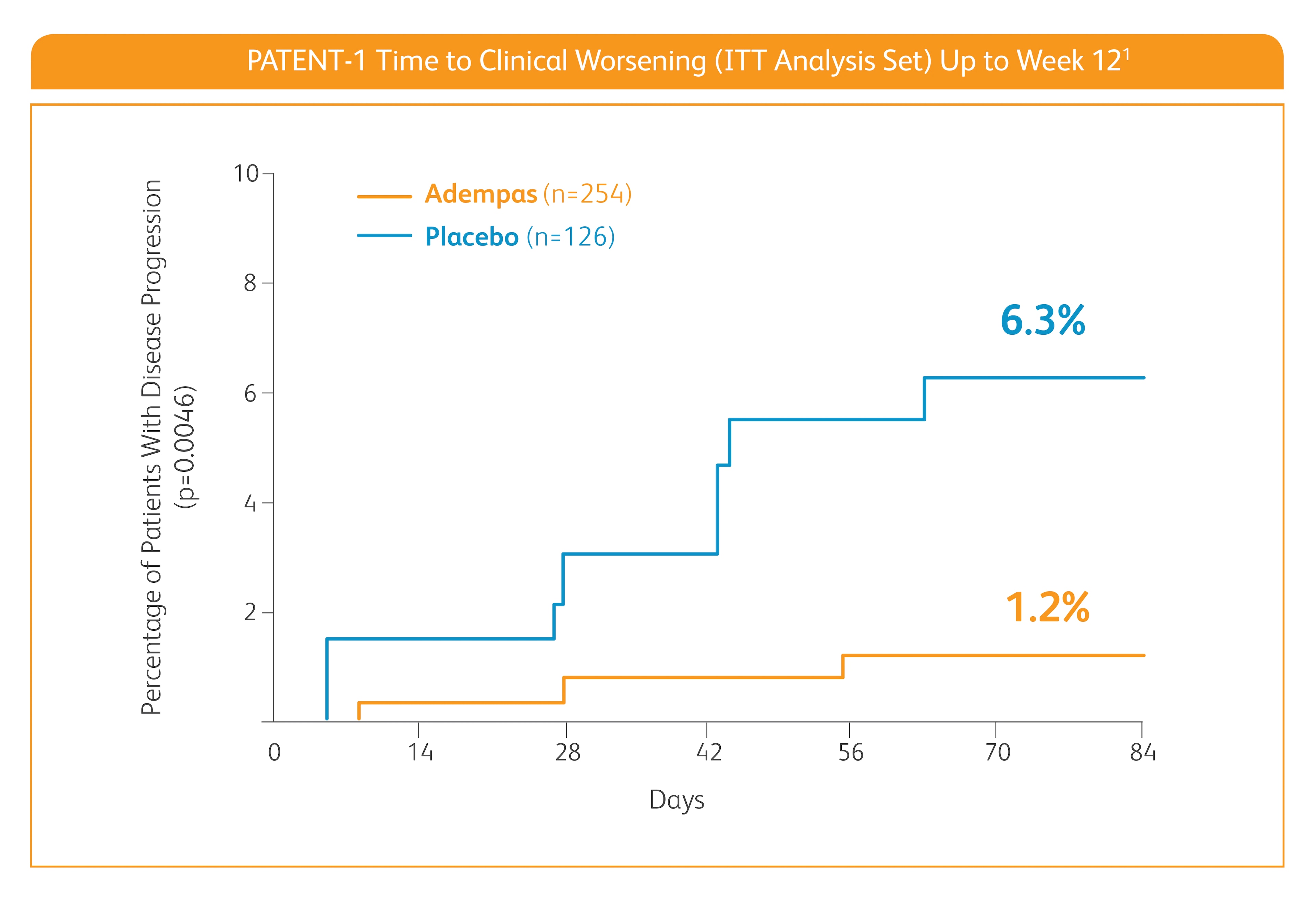
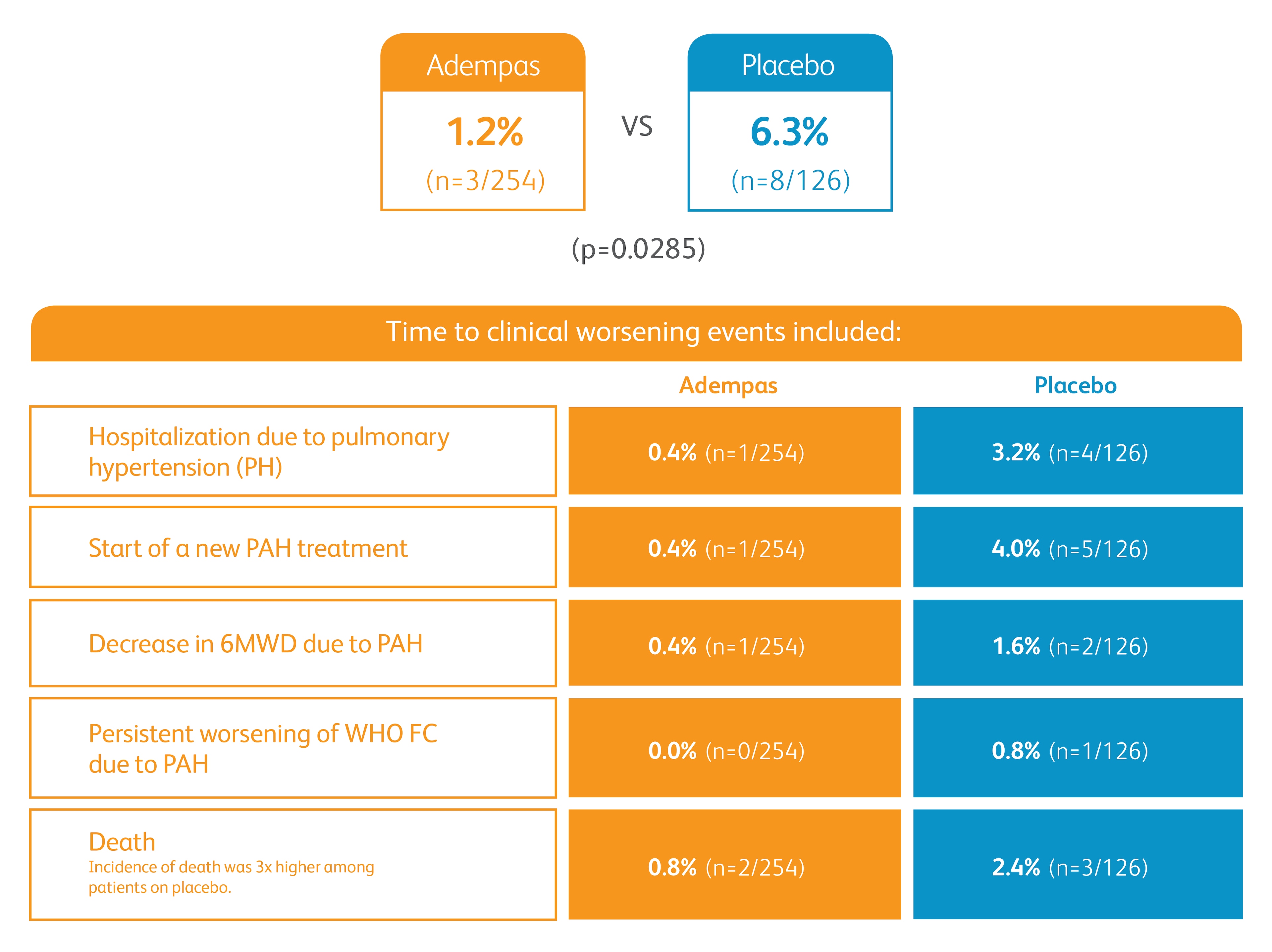
Improvement in time to clinical worsening*
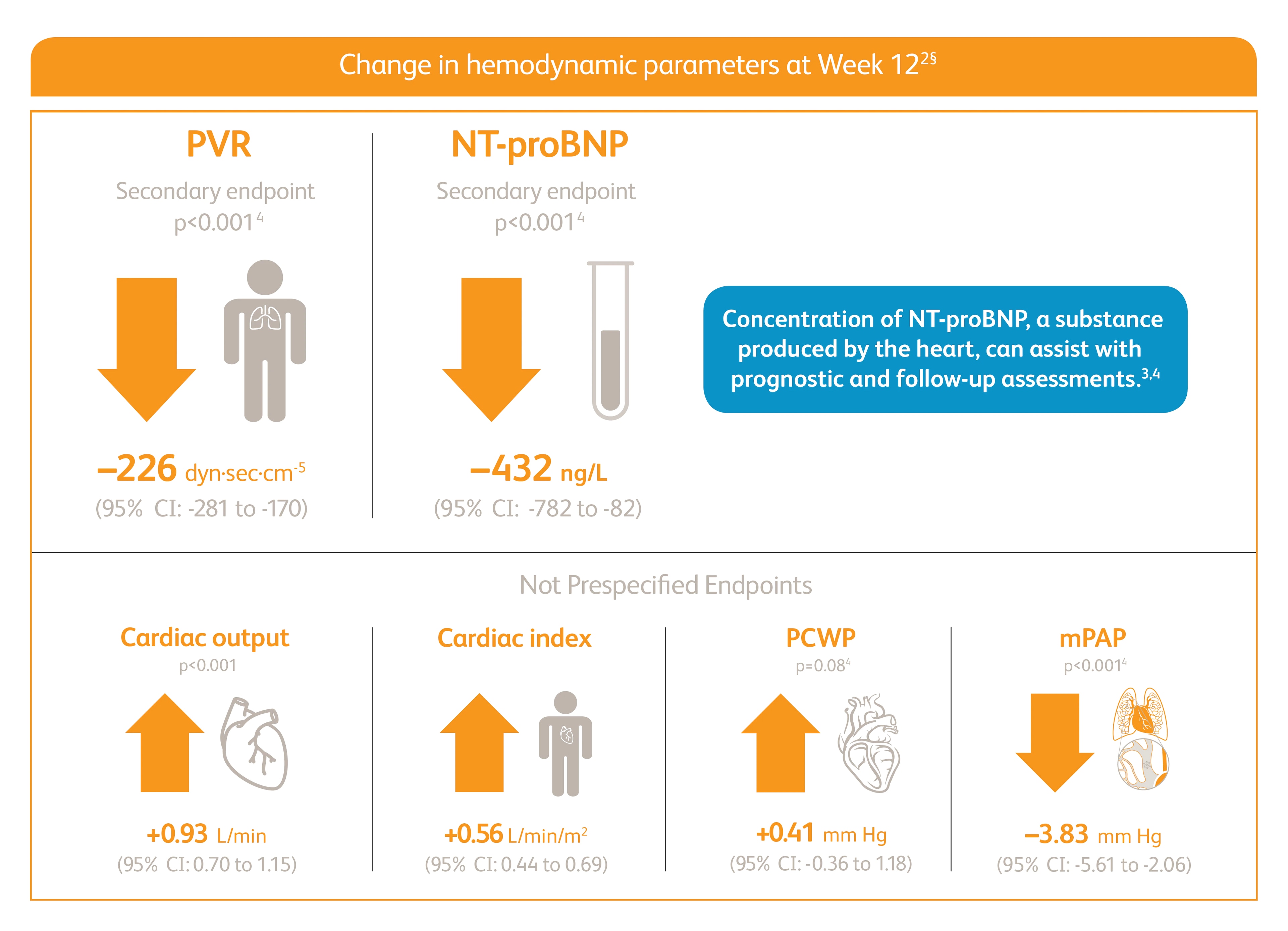
Improvement in PVR and NT-proBNP
6MWD=6-minute walking distance; NT-proBNP=n-terminal prohormone of brain natriuretic peptide; PVR=pulmonary vascular resistance; WHO FC=World Health Organization Functional Class.
*Time to clinical worsening was a combined endpoint defined as death (all-cause mortality), heart/lung transplantation, atrial septostomy, hospitalization due to persistent worsening of pulmonary hypertension, start of new PAH-specific treatment, persistent decrease in 6MWD, and persistent worsening of WHO FC.
PATENT-1 STUDY DESIGN1,2
Pulmonary Arterial Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1 (PATENT-1) was a randomized, double-blind, multinational, multicenter, placebo-controlled, 12-week phase 3 study.
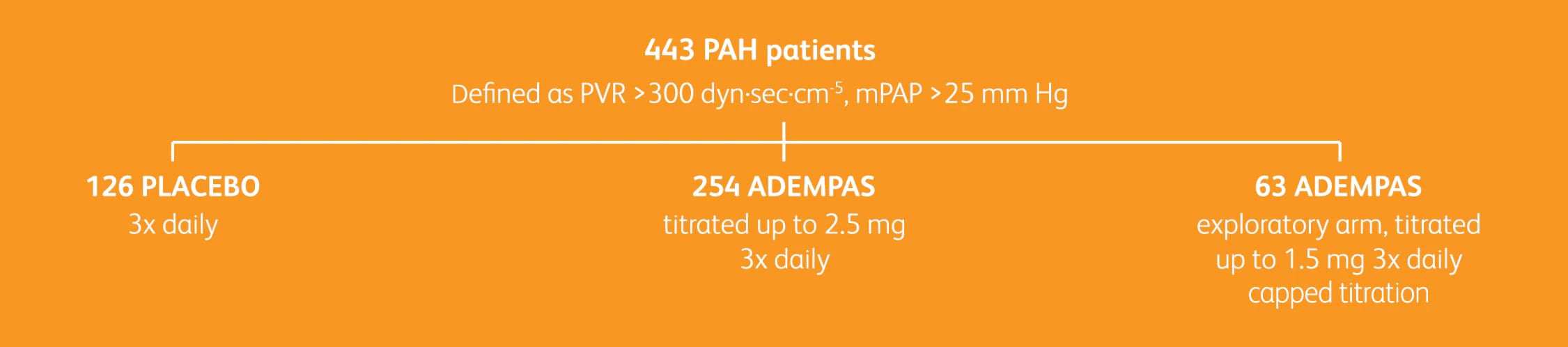
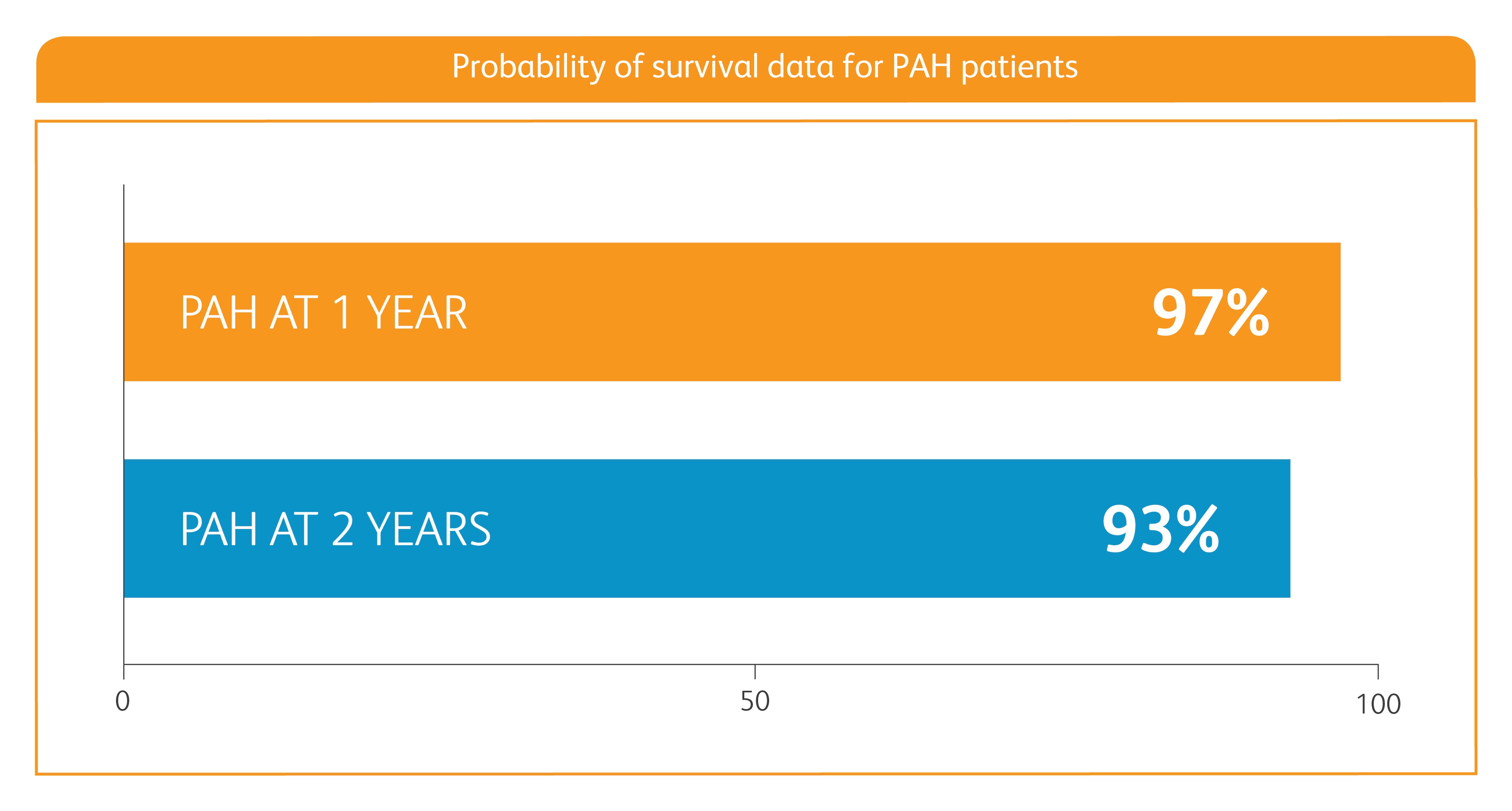
BASELINE CHARACTERISTICS
Mean age: 51 years (~80% female)
PAH cause: Idiopathic (61%), familial (2%), associated with connective tissue disease (25%), congenital heart disease (8%), portal hypertension (3%), or anorexigen/amphetamine use (1%)
WHO FC: II (42%); III (54%)
Mean 6MWD baseline: 363m
Exclusions: Patients with SBP <95 mm Hg

TREATMENT CHARACTERISTICS
Treatment status: Treatment-naïve (50%), pretreated with endothelin receptor antagonist (ERA) (44%), and pretreated with prostacyclin analog (PCA) (6%)
Pretreatment definition: On stable treatment for 3 months with an ERA or PCA; Adempas was combined with these therapies
Concomitant medications: Oral anticoagulants, diuretics, digitalis, calcium channel blockers, and oxygen were allowed

DOSING CHARACTERISTICS
Initiation: 1 mg 3x daily
Groups: Adempas at 2.5 mg 3x daily; Adempas at 1.5 mg 3x daily; placebo
Titration: ~75% were titrated to 2.5 mg 3x daily by Week 12
Adempas titrated every 2 weeks based on SBP and signs or symptoms of hypotension
FC=functional class; mPAP=mean pulmonary arterial pressure; SBP=systolic blood pressure.
- Adempas Prescribing Information. Whippany, NJ. Bayer Pharmaceuticals Inc., 2021.
- Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330-340.
- Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37(1):67-119.
- McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25):D73-D81.