IF YOUR PATIENTS ARE NOT AT GOAL, CONSIDER ADEMPAS
Adempas demonstrated efficacy for CTEPH adult patients in both WHO Functional Class II and III by 3 parameters1
Improvement in 6MWD
Improvement in WHO FC
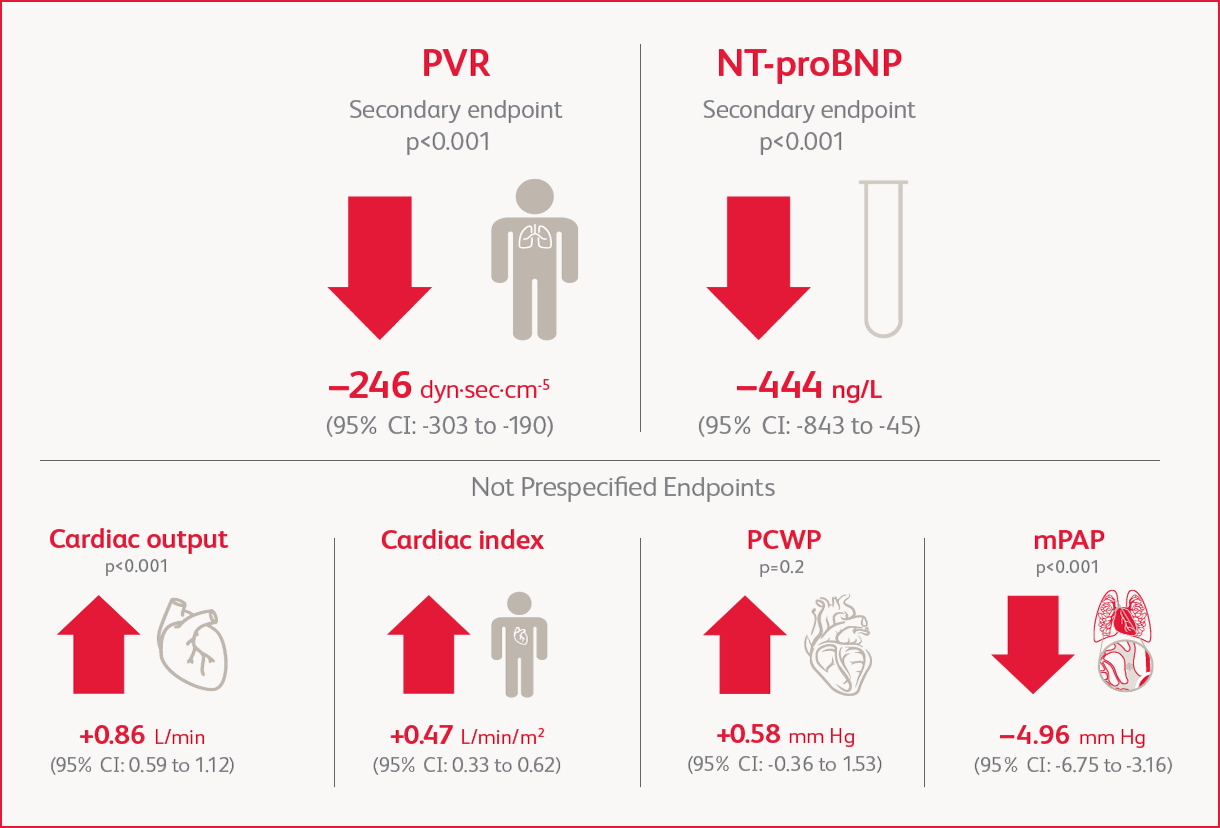
Improvement in PVR and NT-proBNP
6MWD=6-minute walking distance; CTEPH=chronic thromboembolic pulmonary hypertension; NT-proBNP=N-terminal pro-brain natriuretic peptide; PVR=pulmonary vascular resistance; WHO FC=World Health Organization Functional Class.
CHEST STUDY DESIGN1,2
Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1 (CHEST-1) was a randomized, double-blind, multinational, multicenter, placebo-controlled, 16-week phase 3 study.

Results in CTEPH: 6MWD Over 16 Weeks
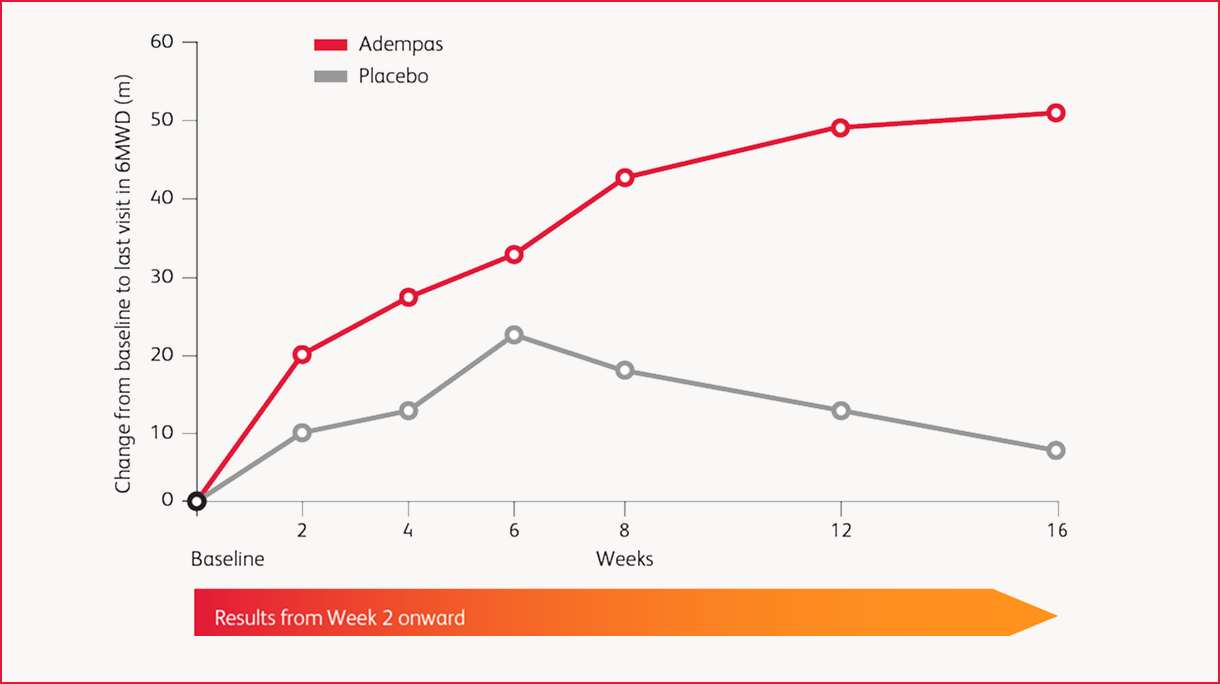
improvement (mean) in 6-minute walk distance (6MWD) over placebo at Week 16 (95% confidence interval (CI): 25 m-67 m; p<0.0001) for adults with CTEPH (WHO Group 4).
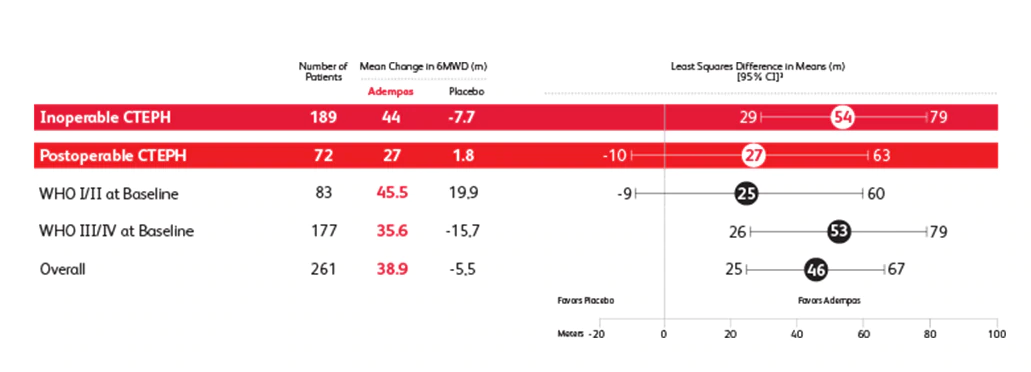
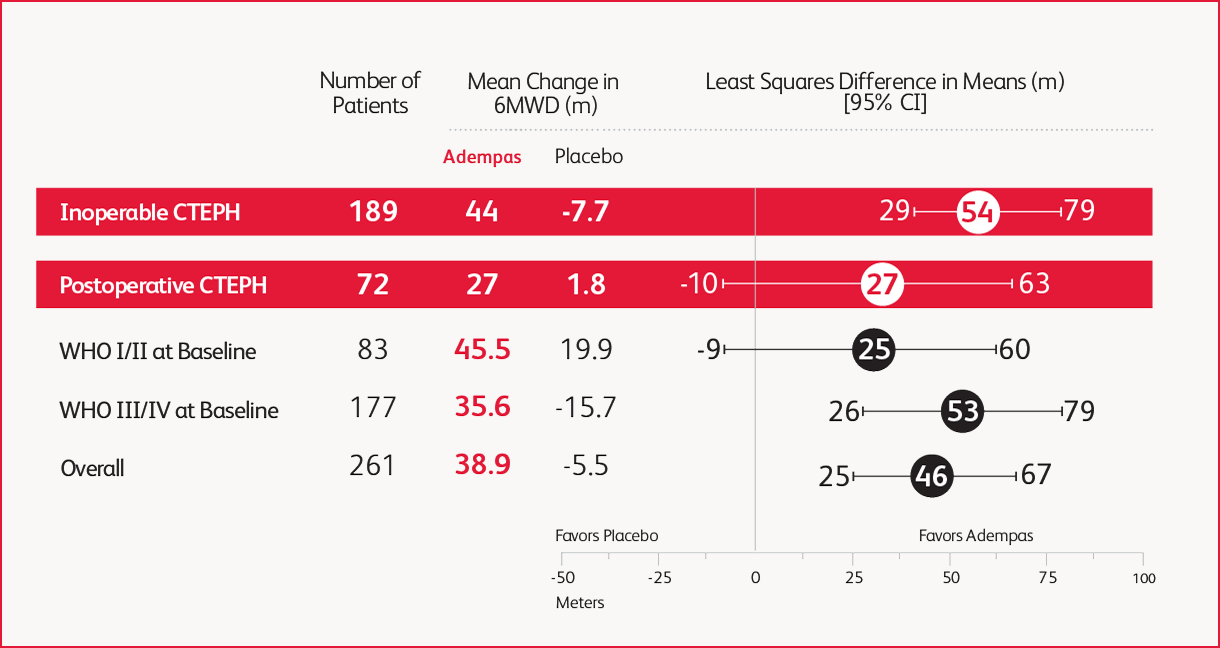
Prespecified Subgroup: Mean Change From Baseline
Change in WHO Functional Class in the 16-Week Trial
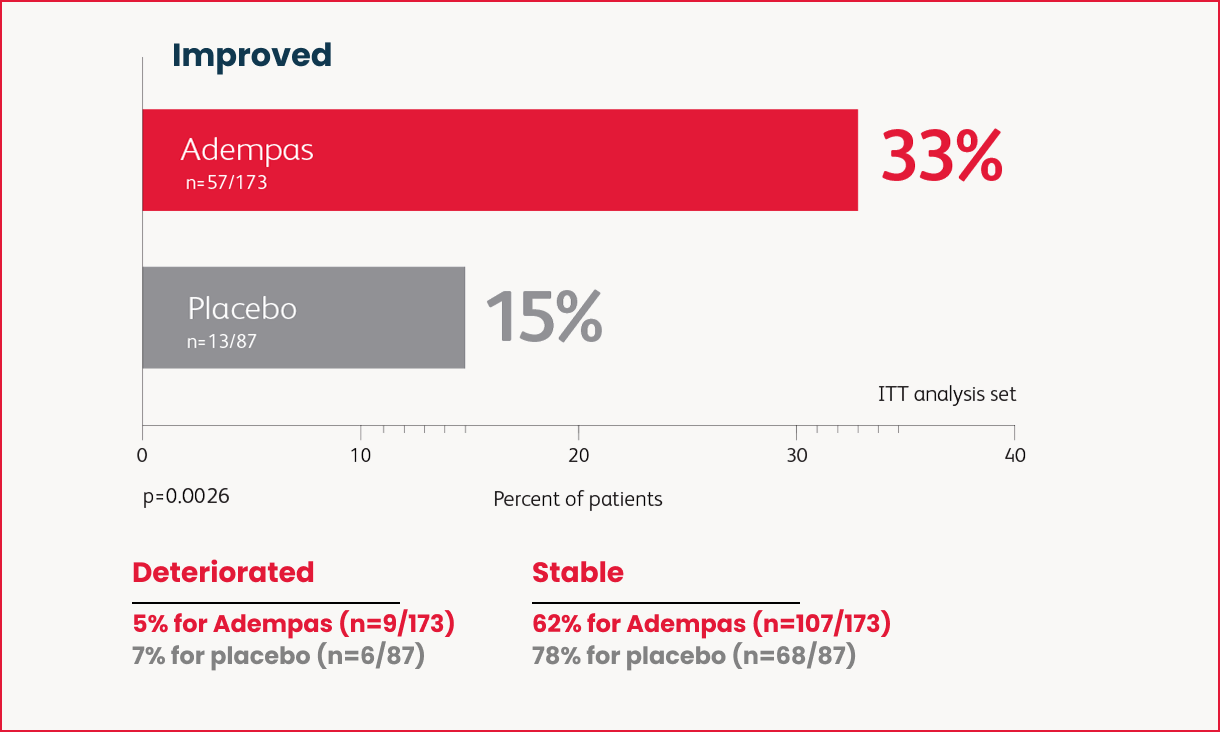
as many patients improved WHO FC vs placebo
Change in Hemodynamic Parameters at Week 16
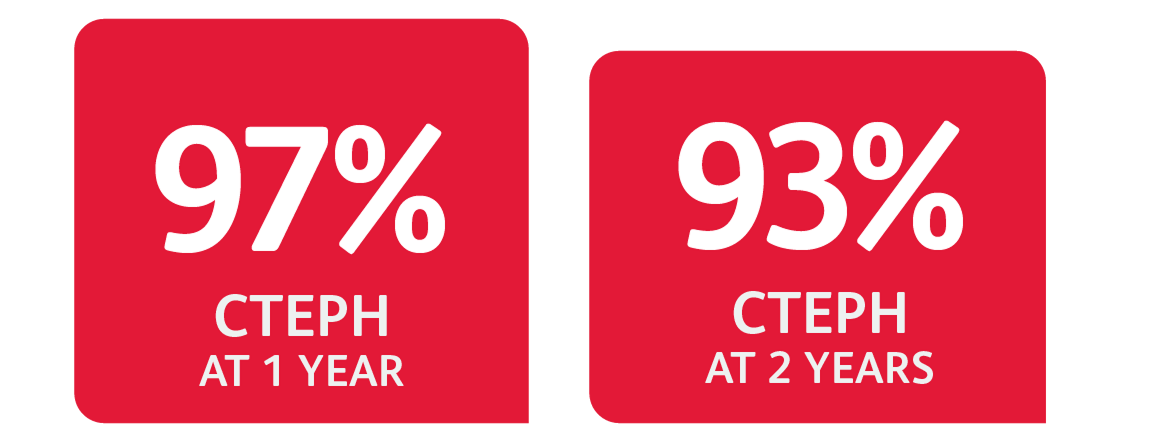
Probability of Survival Data for CTEPH Patients
§Data from CHEST-2 open-label extension study.
An open-label extension study (CHEST-2) included 237 patients who had completed CHEST-1.
At the cut-off date in the CHEST-2 study, the mean treatment duration for the total population was 1077 days (±433).
Without a control group, these data must be interpreted cautiously.
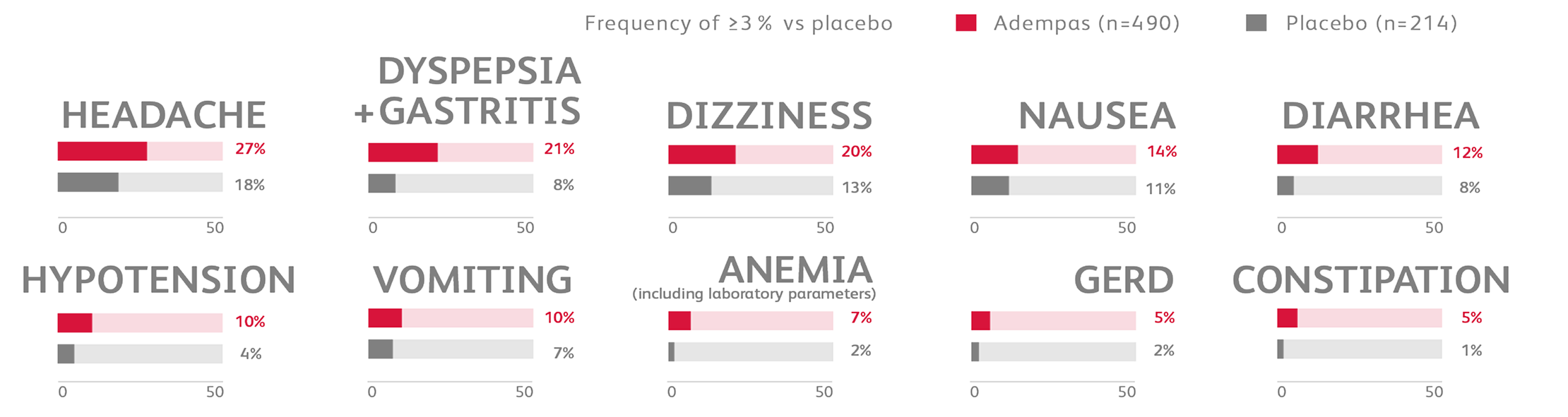
Other events that were seen more frequently in Adempas compared to placebo and potentially related to treatment were:
- Palpitations
- Nasal congestion
- Epistaxis
- Dysphagia
- Abdominal distention
- Peripheral edema
Help your patients take Adempas properly.
Learn more here.
- Adempas Prescribing Information. Whippany, NJ. Bayer Pharmaceuticals Inc., 2021.
- Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319-329.
- Clinical Study Report A62508. Data on File. Bayer HealthCare, Inc; Whippany, NJ.