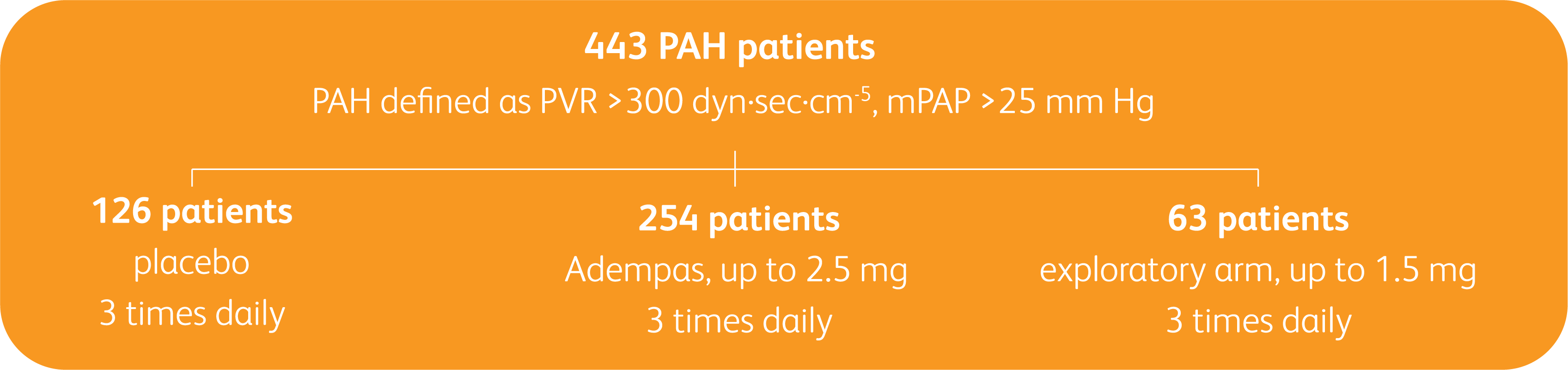
PATENT-1 Study Design
PIVOTAL TRIAL IN ADULT PATIENTS WITH PAH (WHO GROUP 1)1
Randomized, double-blind, multinational, placebo-controlled, 12-week, phase 3 study
Primary endpoint1
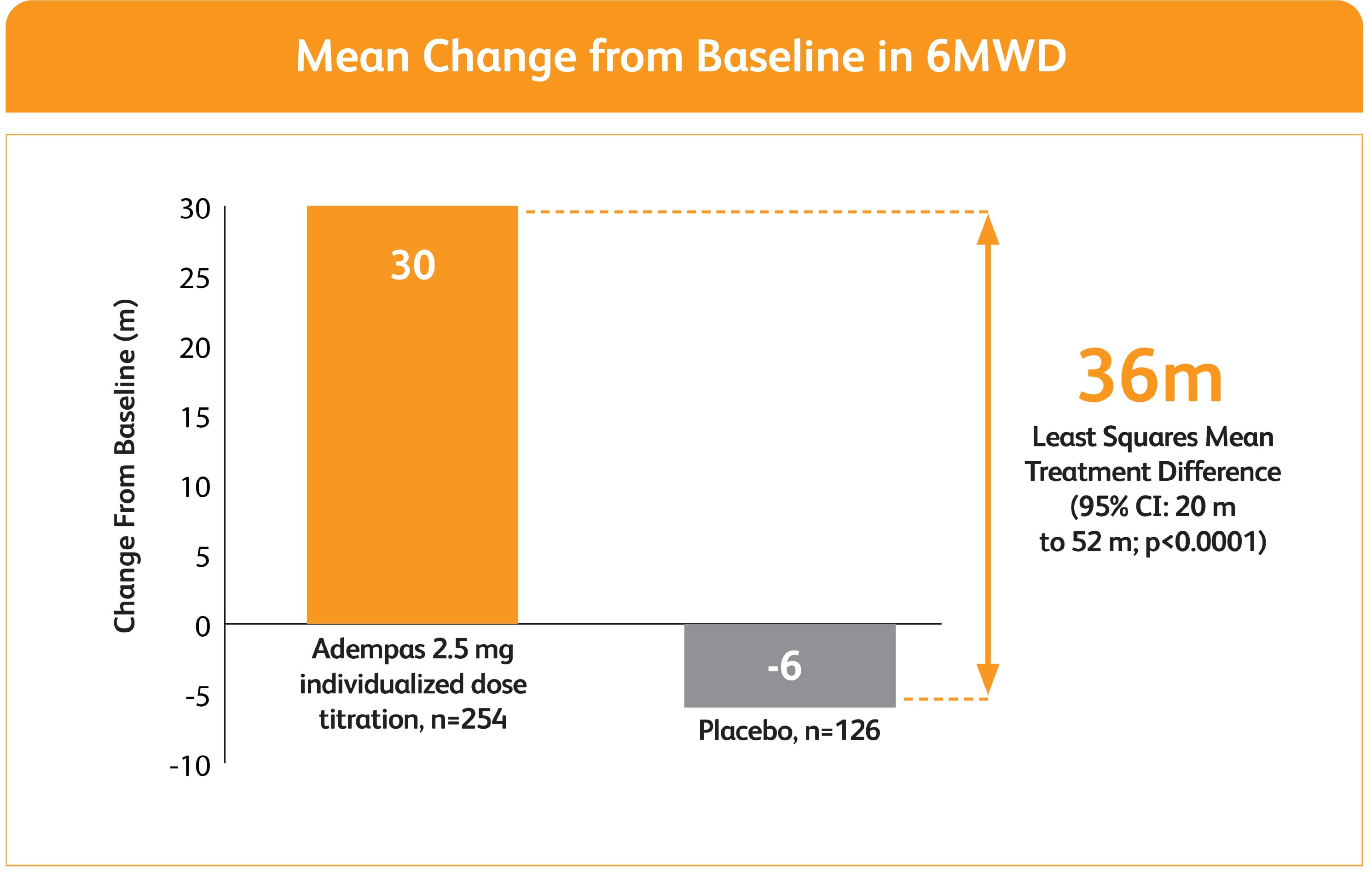
- Change in 6MWD from baseline to Week 12
Baseline characteristics1
- Mean age: 51 years (approximately 80% female)
- PAH etiologies: idiopathic (61%), associated with connective tissue disease (25%), congenital heart disease (8%), portal hypertension (3%), familial (2%), or anorexigen or amphetamine use (1%)
- Mean 6MWD was 363 m
- Concomitant medications: oral anticoagulants, diuretics, digitalis, calcium channel blockers, and oxygen were allowed
- Patient population: 50% treatment naïve, 44% pretreated with an endothelin receptor antagonist (ERA), and 6% pretreated with a prostacyclin analog (PCA)
- The majority of patients had WHO FC II (42%) or III (54%) at baseline
- Patients with systolic blood pressure <95 mm Hg were excluded
6MWD = 6-minute walk distance; m = meters; mPAP = mean pulmonary arterial pressure; PAH = pulmonary arterial hypertension; PATENT-1 = Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator Trial; PVR = pulmonary vascular resistance; WHO FC = World Health Organization Functional Class.
PATENT-1 Results
IMPROVEMENT IN 6MWD WAS OBSERVED AT 2 WEEKS AND CONTINUED THROUGH 12 WEEKS1
SIGNIFICANT IMPROVEMENT IN 6MWD AT WEEK 12
Adempas can be used as monotherapy or in combination
- In WHO FC II and III—in combination with ERAs
- As monotherapy—in combination with PCAs
50% MORE PATIENTS IMPROVED WHO FC ON ADEMPAS VS PLACEBO
CHANGE IN WHO FC IN THE 12-WEEK PATENT-1 STUDY
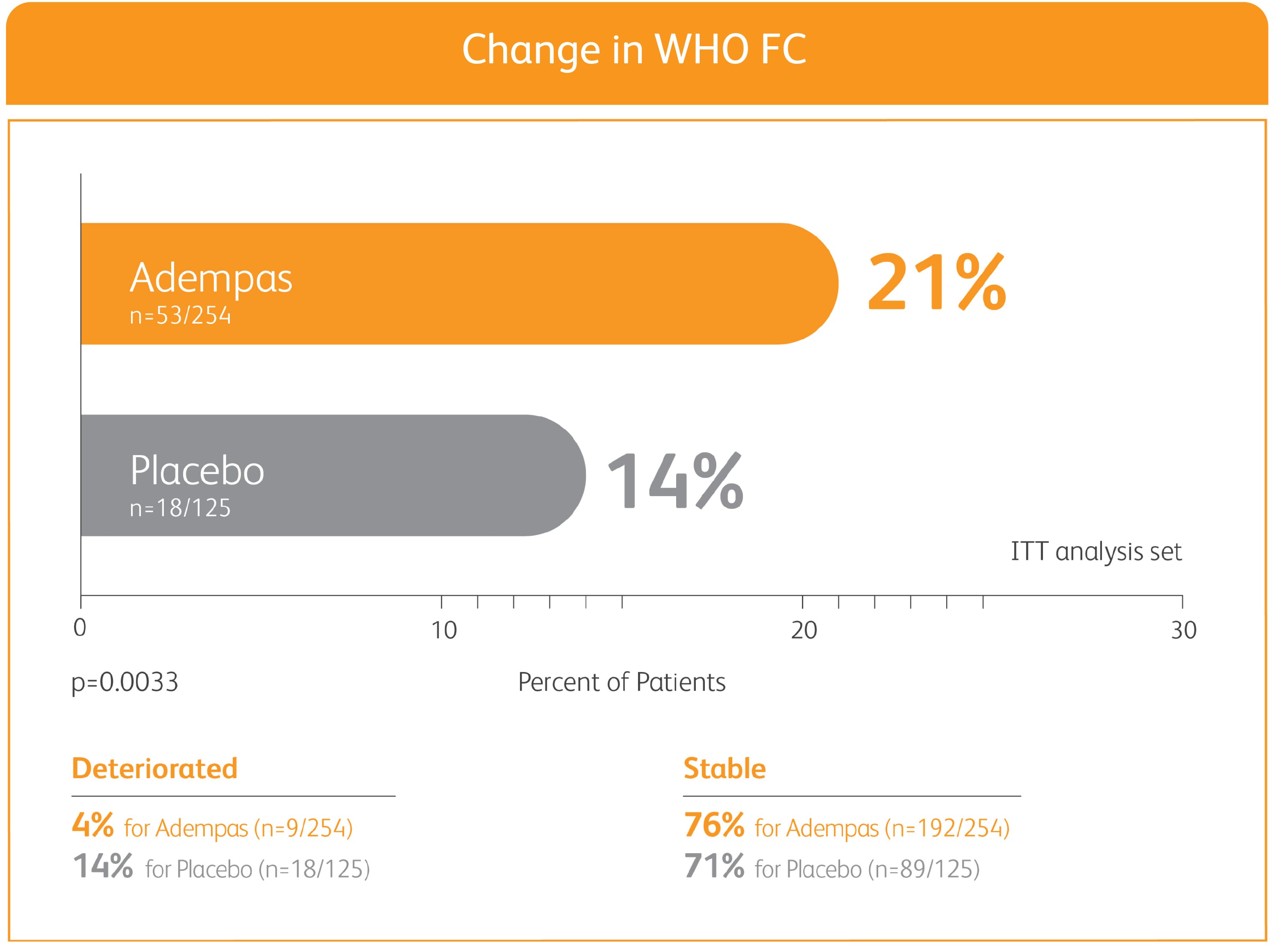
ITT = intention-to-treat; WHO FC = World Health Organization Functional Class.
ADEMPAS IMPROVED PVR AND NT-proBNP AT WEEK 12
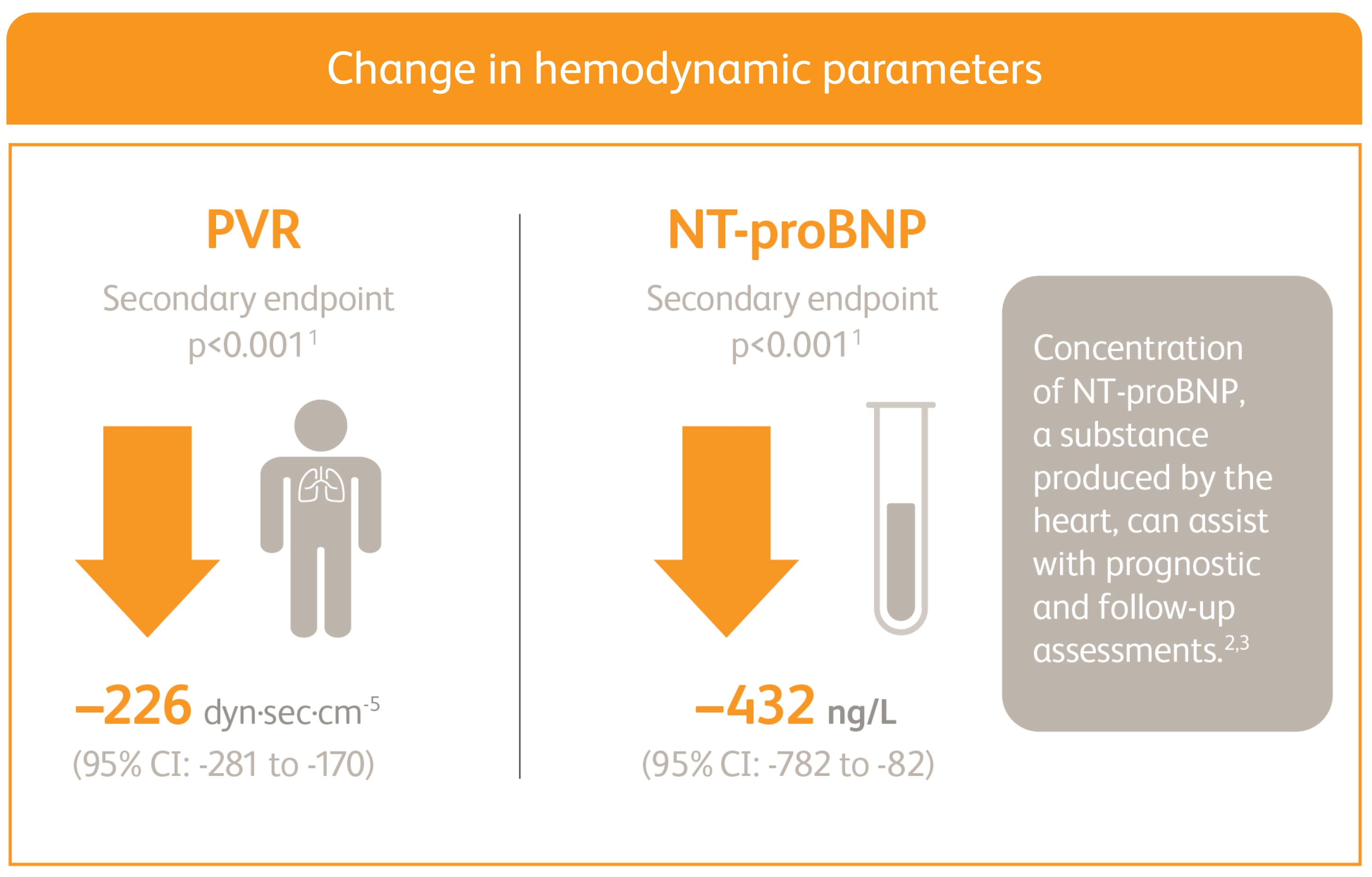
Right heart catheterization was performed at the beginning and end of the study period in 339 patients.
*Placebo-adjusted mean change from baseline.
NT-proBNP = n-terminal pro-brain natriuretic peptide.
CLINICAL WORSENING EVENTS UP TO 12 WEEKS
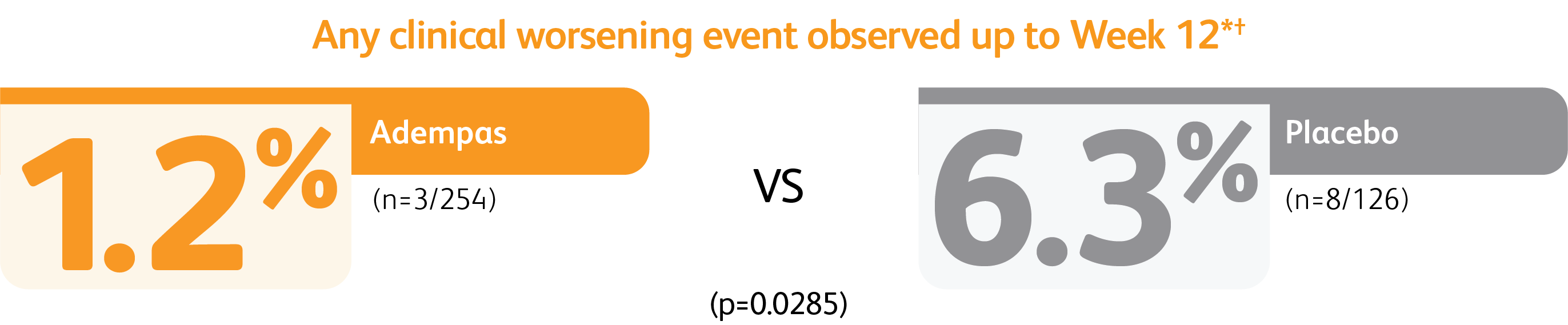
*Time to clinical worsening was a combined endpoint defined as death (all-cause mortality), heart/lung transplantation, atrial septostomy, hospitalization due to persistent worsening of PH, start of new PAH-specific treatment, persistent decrease in 6MWD, and persistent worsening of WHO Functional Class.
†Patients may have had more than one event of clinical worsening.
PATENT-1 Study Design
PATENT-1: A RANDOMIZED, DOUBLE-BLIND, MULTINATIONAL, MULTICENTER, PLACEBO-CONTROLLED, 12-WEEK, PHASE 3 STUDY
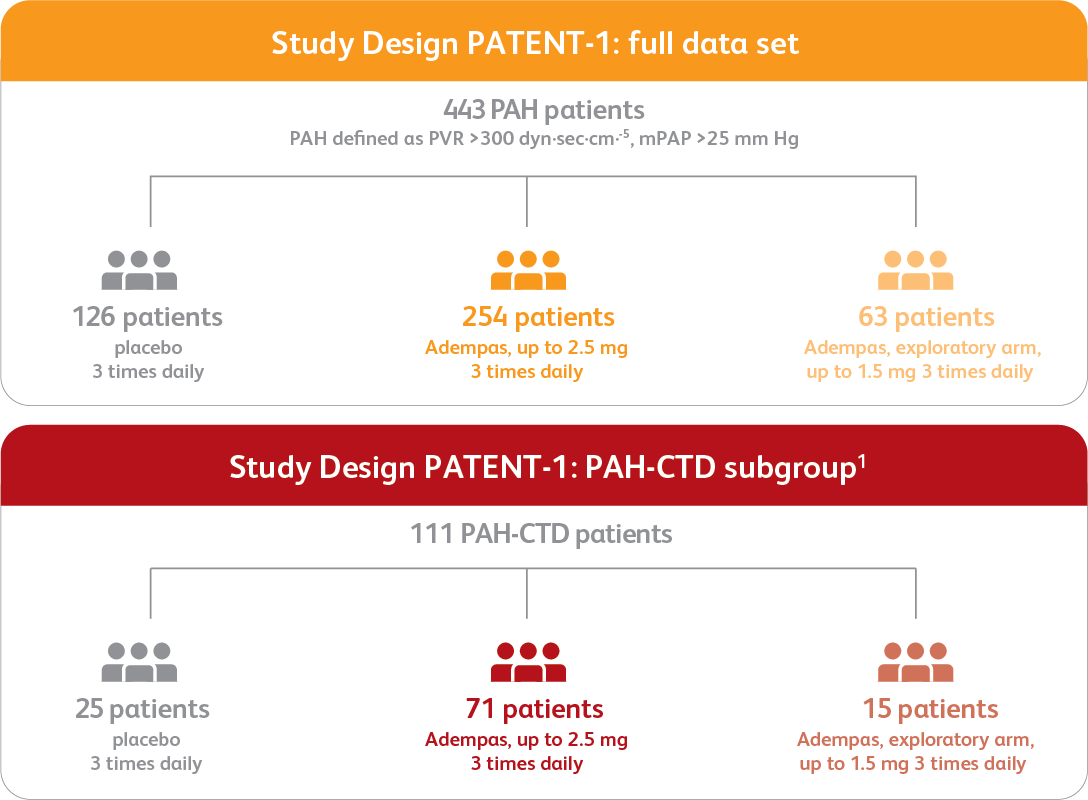
Efficacy data are shown for the Adempas 2.5 mg and placebo arms.
Data from the PAH-CTD subgroup analysis are exploratory as PATENT-1 was not designed to show statistically significant differences in subgroup populations. The primary efficacy analysis was performed on data from the modified intent-to-treat population (all patients who were randomized and received one or more doses of the study drug). The data presented here are observed values and are analyzed descriptively; observed values were used due to the small sample size and the retrospective, exploratory nature of the analysis.
BASELINE CHARACTERISTICS2,3
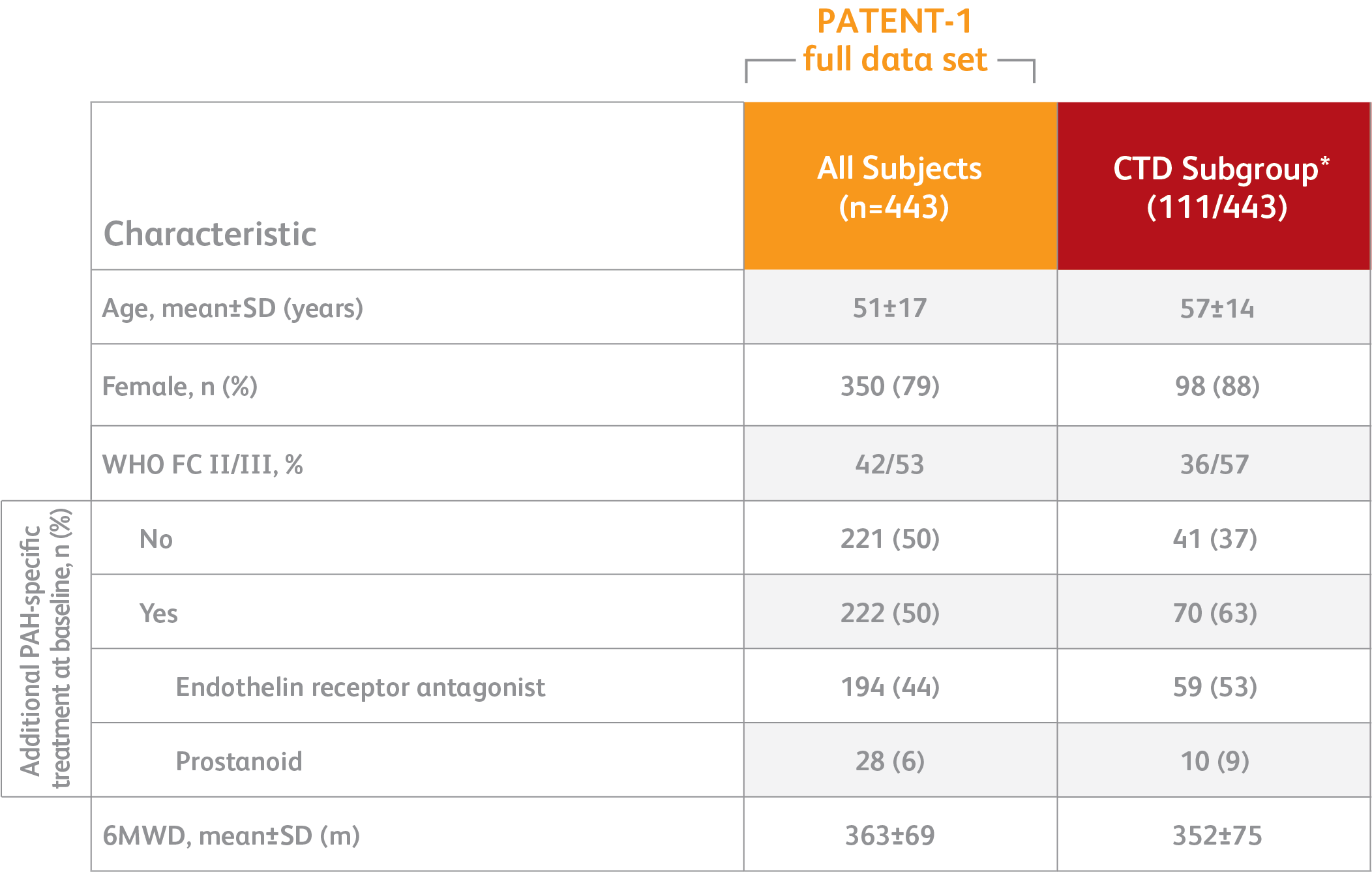
*Exploratory subgroup analysis.
- PAH defined as: PVR >300 dyn·sec·cm-5, mPAP >25 mm Hg
- PATENT-1 PAH etiologies: idiopathic (61%), familial (2%), associated with connective tissue disease (25%), congenital heart disease (8%), portal hypertension (3%), or anorexigen or amphetamine use (1%)
- Concomitant medications: oral anticoagulants, diuretics, digitalis, calcium channel blockers, and oxygen were allowed
- Patients with systolic blood pressure <95 mm Hg were excluded
6MWD = 6-minute walk distance; m = meters; mm Hg = millimeters mercury; mPAP = mean pulmonary arterial pressure; PATENT-1 = Pulmonary Arterial Hypertension Soluble Guanylate Cyclase-Stimulator Trial; PVR = pulmonary vascular resistance; WHO FC = World Health Organization Functional Class.
PATENT-1: 6MWD at Week 12 based on patient characteristics
MEAN TREATMENT DIFFERENCE IN CHANGE FROM BASELINE TO WEEK 12*
IN 6MWD BY PRESPECIFIED SUBGROUPS4
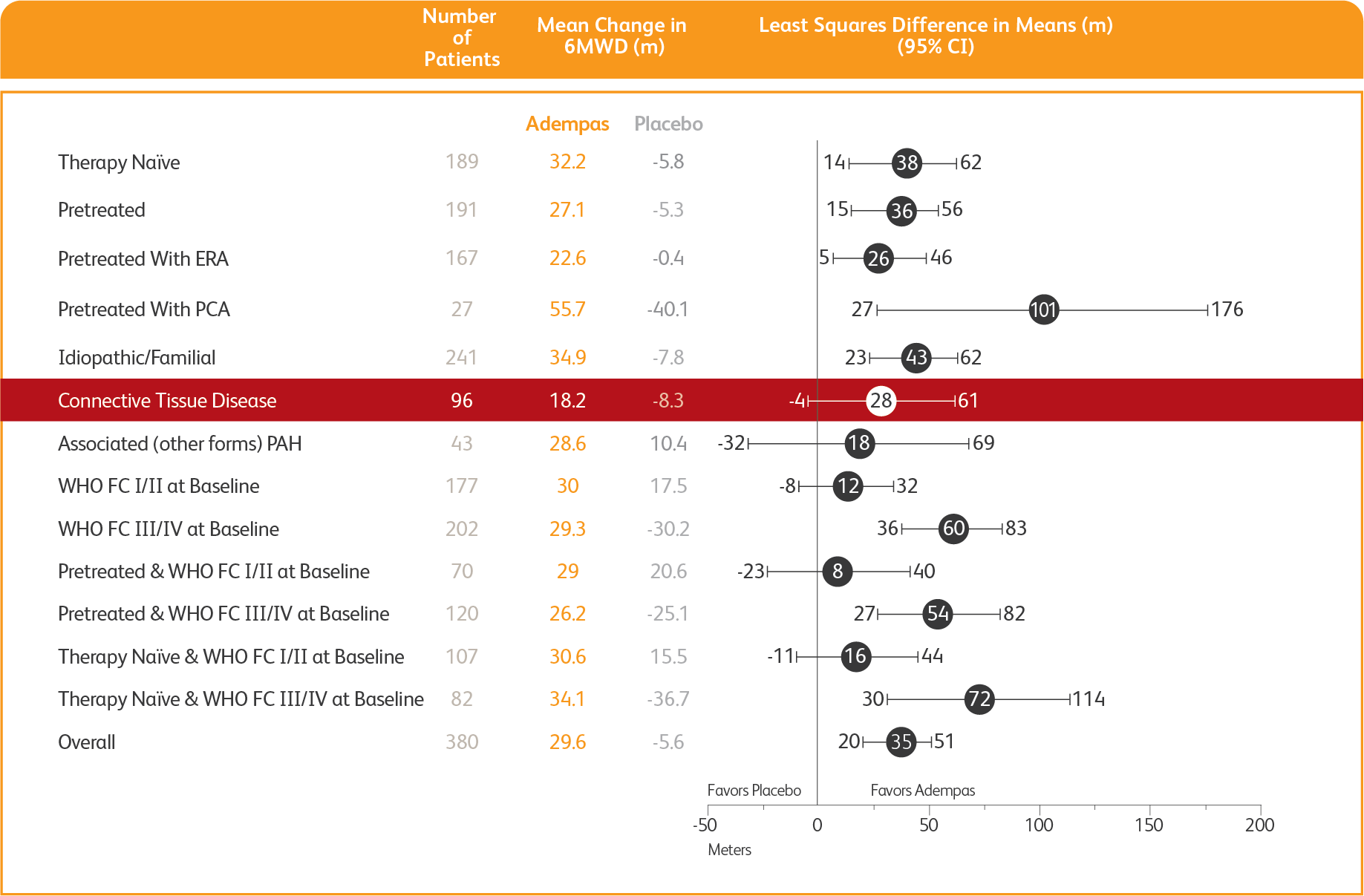
*Week 12/last observation until Week 12.
ERA = endothelin receptor antagonist; PCA = prostacyclin analogues.
PATENT-1 Results in the CTD Subgroup
The data in these analyses should be considered exploratory and not designed to detect statistically significant differences in subgroups. The analysis is limited due to the post hoc sub-classification of patients with PAH-CTD by patients’ medical histories using MedDRA terms. The low patient numbers in the PAH-SSc and PAH-other defined CTD subgroups should be taken into consideration when interpreting the data.3
CHANGE IN 6MWD FROM BASELINE TO WEEK 121,3
EXPLORATORY POST HOC SUBGROUP ANALYSIS
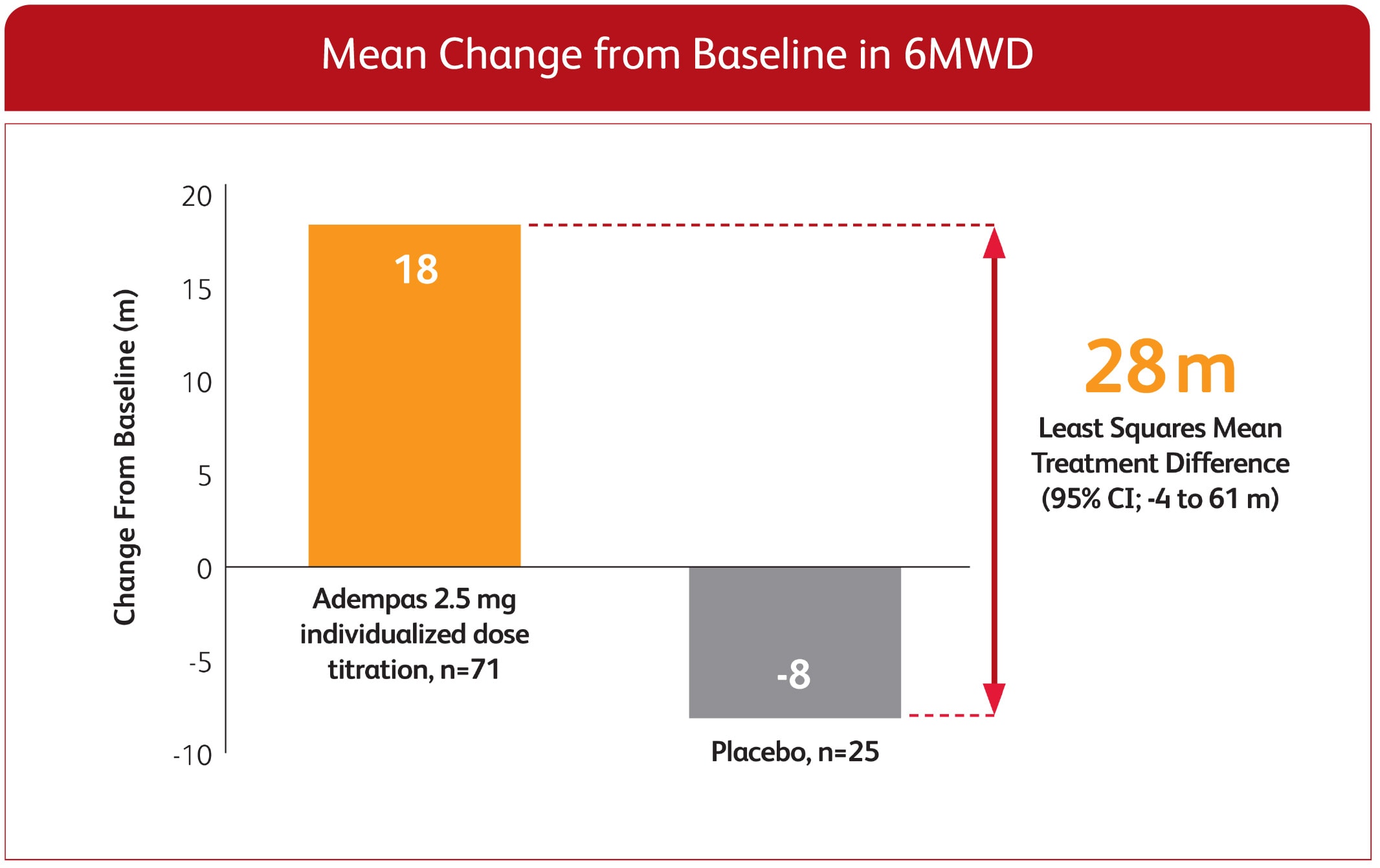
*Week 12/last observation until Week 12.
ITT population.
CHANGE IN WHO FC FROM BASELINE TO 12-WEEK PATENT-1 STUDY1
EXPLORATORY POST HOC SUBGROUP ANALYSIS
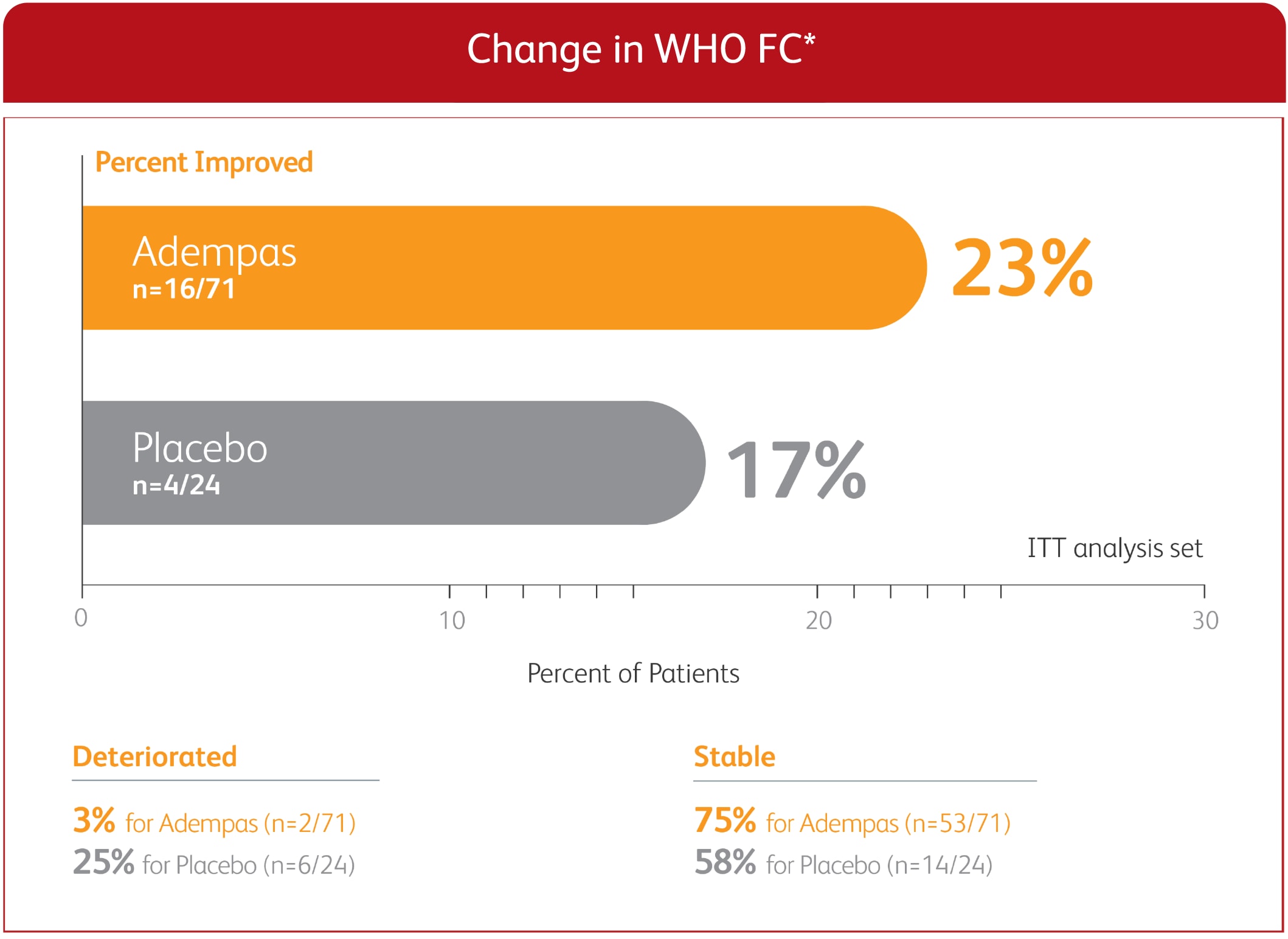
*Week 12/last observation until Week 12. ITT population. Missing values, where the patient withdrew or dies, were imputed at Week 12 according to the last observed value.
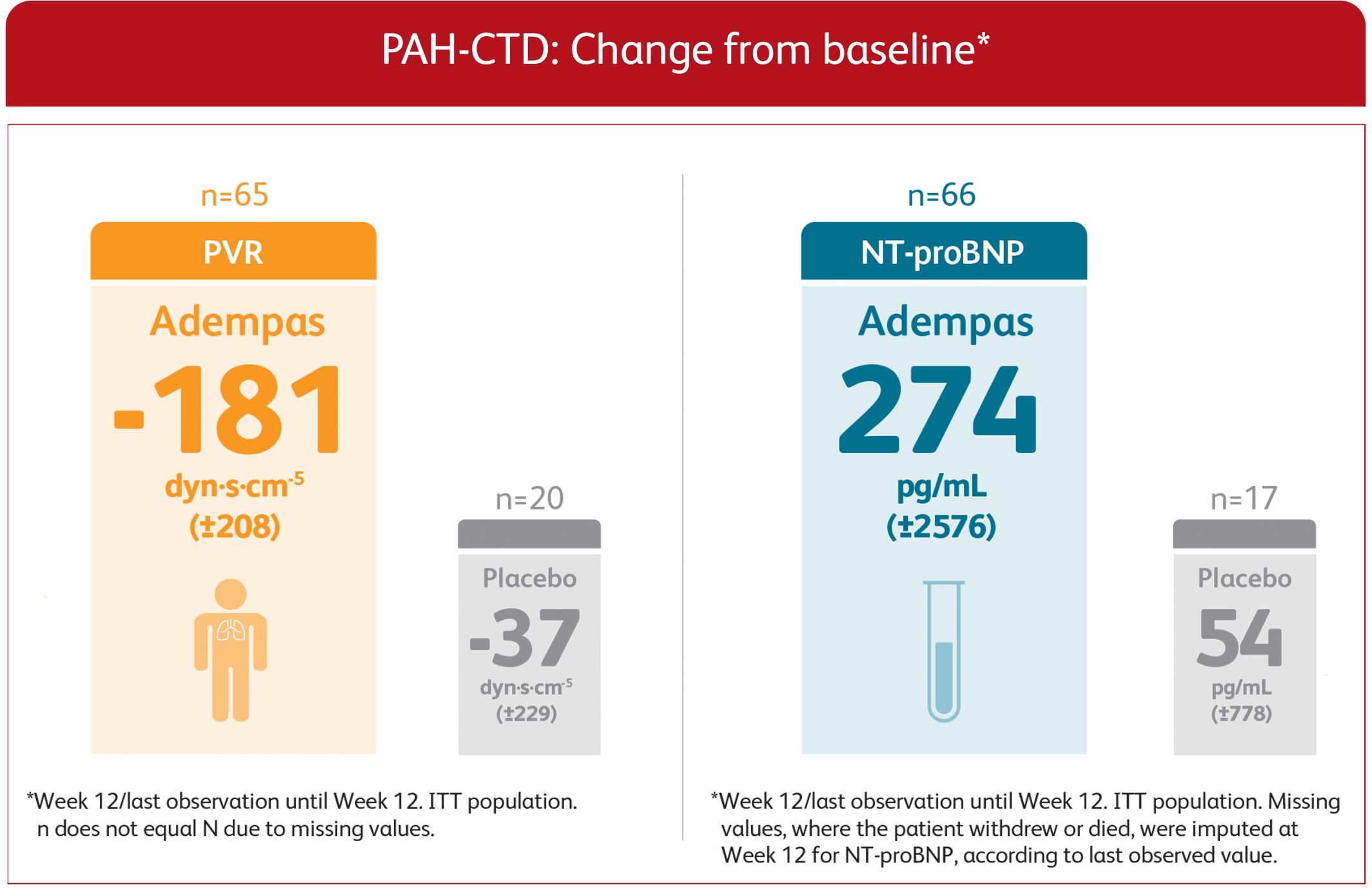
PVR AND NT-proBNP IN PATENT-1 PAH-CTD PATIENTS TREATED WITH ADEMPAS VS PLACEBO AT WEEK 123
EXPLORATORY POST HOC SUBGROUP ANALYSIS
CLINICAL WORSENING EVENTS UP TO WEEK 12
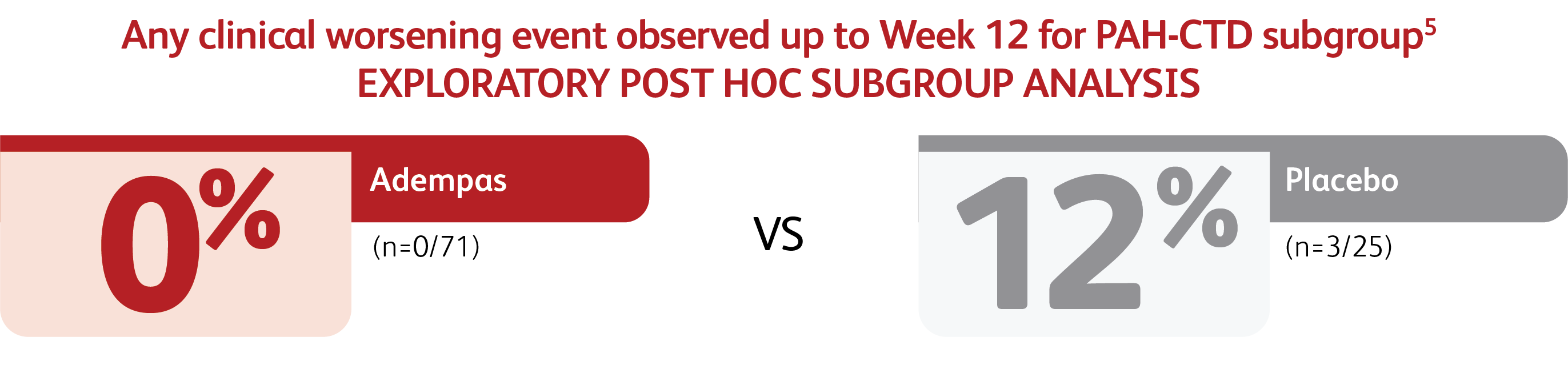
*Time to clinical worsening was a combined endpoint defined as death (all-cause mortality), heart/lung transplantation, atrial septostomy, hospitalization due to persistent worsening of PH, start of new PAH-specific treatment, persistent decrease in 6MWD, and persistent worsening of WHO Functional Class.
†Patients may have had more than one event of clinical worsening.
LONG-TERM DATA: PROBABILITY OF SURVIVAL AT 2 YEARS
An open-label extension study (PATENT-2) included 396 patients who had completed PATENT-1. At the cut-off date in the PATENT-2 study, the mean treatment duration for the total population was 1146 days (±479). The probabilities of survival at 1 and 2 years were 97% and 93%, respectively. Without a control group, these data must be interpreted cautiously.

PREVALENCE OF PAH-CTD
- PAH associated with CTD is the second most prevalent type of PAH after idiopathic PAH (IPAH) in the Western world6
- PAH associated with CTD represents about 30% of the PAH population, as described in the REVEAL registry7
- PAH-CTD includes systemic scleroderma (SSc), systemic lupus erythematosus (SLE), and mixed CTD6
– About 10%-18% of patients with SSc or SLE develop PAH over their lifetime8,9
HOW IS PAH-CTD DIAGNOSED?
- Resting echocardiography is recommended as a screening test in asymptomatic patients with SSc followed by annual screening with echocardiography, diffusing capacity of the lungs for carbon monoxide (DLCO), and biomarkers6,10
- Treatment of underlying condition with medication or right heart catheterization (RHC) is recommended in cases of suspected PAH associated with CTD6,10
RISK FACTORS FOR PAH IN PATIENTS WITH CTD INCLUDE:
- Female gender6
- Older age6
- Raynaud’s disease8
- Active renal disease8
- Some types of vasculitis (ie, digital gangrene, cutaneous vasculitis)8
- Limited cutaneous systemic sclerotic disease of >5 years’ duration11
- Low diffusing capacity of the lung for carbon monoxide (DLCO)11
- Anti-centromere antibody positive11
ITT = intention to treat; NT-proBNP = n-terminal pro-brain natriuretic peptide; SLE = systemic lupus erythematosus; SSc = systemic scleroderma.
References:
- Ghofrani H-A, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330-340.
- Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37(1):67-119.
- McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25):D73-D81.
References:
- Humbert M, Coghlan JG, Ghofrani H-A, et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann Rheum Dis. 2017;76(Suppl):1-19.
- Ghofrani H-A, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330-340.
- Humbert M, Coghlan JG, Ghofrani H-A, et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann Rheum Dis. 2017;76(2):422-426.
- Ghofrani H-A, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(Suppl):1-27.
- Data on File.
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;00:1-114.
- Farber HW, Miller DP, Poms AD, et al. Five-Year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148(4):1043-1054.
- Tselios K, Gladman D, Urowitz MB. Systemic lupus erythematosus and pulmonary arterial hypertension: links, risks, and management strategies. Open Access Rheumatol. 2017;9:1-9.
- Thakkar V, Lau EMT. Connective tissue disease-related pulmonary arterial hypertension. Best Pract Res Clin Rheumatol. 2016;30:22-38.
- Khanna D, Gladue H, Channick R, et al. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum. 2013;65:3194-3201.
- Condliffe R, Howard LS. Connective tissue disease-associated pulmonary arterial hypertension. F1000Prime Rep. 2015;7:06.



