WHAT IS CTEPH?
CTEPH is a form of pulmonary hypertension (PH) and is designated as WHO Group 4. Diagnosis of CTEPH is based on the following after 3 months of anticoagulation1-3
- PVR ≥3 WU or 240 dyn•sec•cm-5
- Mean pulmonary arterial pressure (mPAP) >20 mm Hg
- Pulmonary artery wedge pressure (PAWP) ≤15 mm Hg
- The presence of multiple chronic/ organized occlusive thrombi/emboli in the elastic pulmonary arteries as indicated by mismatched perfusion defects on lung scan and specific signs of occlusion on imaging
CTEPH=chronic thromboembolic pulmonary hypertension; WHO=World Health Organization; WU=Wood units.
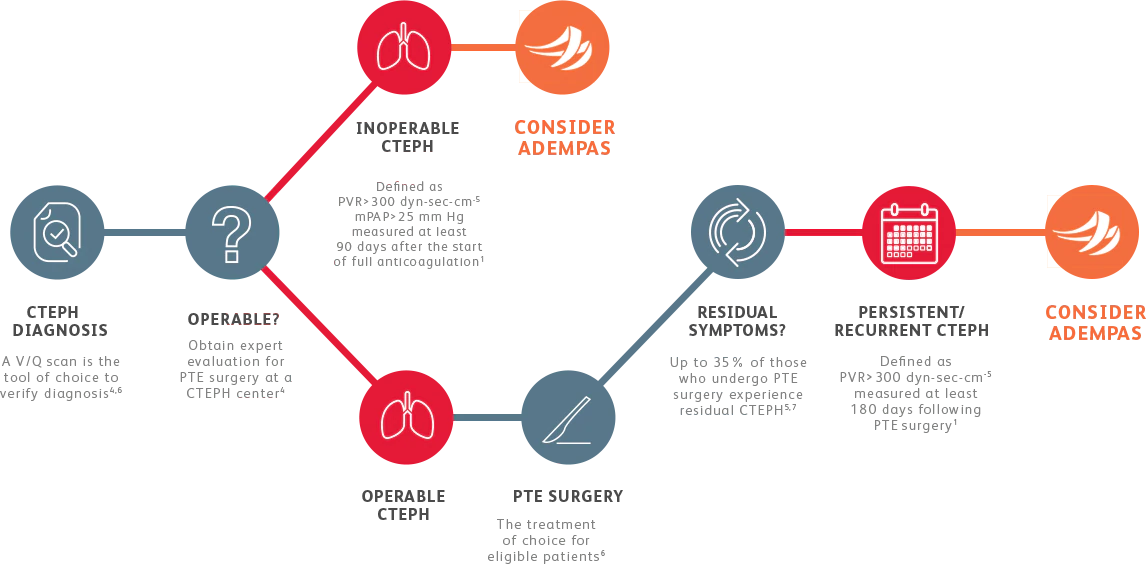
EVALUATING CTEPH PATIENTS FOR TREATMENT
All patients should be evaluated by CTEPH experts for pulmonary thromboendarterectomy (PTE) surgery.4
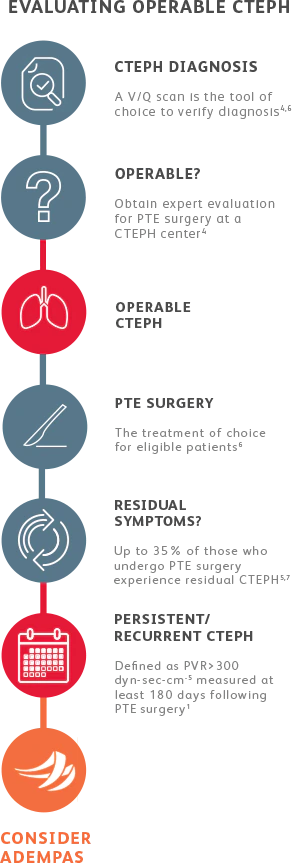
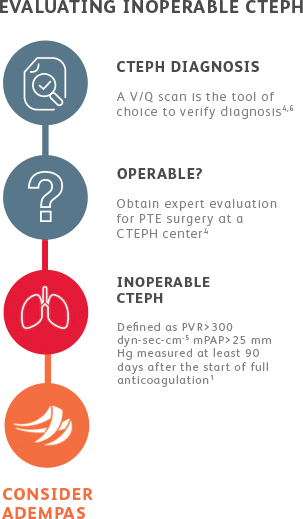
Adempas is the first and only FDA-approved therapy to treat adults with inoperable or persistent/ recurrent CTEPH.
Adempas was predominantly studied in WHO functional class II-III patients.1
V/Q=ventilation/perfusion.

PATHOGENESIS
CTEPH may be caused by either single or recurrent pulmonary emboli that incompletely resolve, resulting in endothelialized residua that obstruct or significantly narrow pulmonary arteries.8
CTEPH results from persistent macrovascular obstruction, often associated with vasoconstrictor response, which leads to a secondary small vessel arteriopathy. Small vessel arteriopathy is characterized by medial hypertrophy, intimal proliferation and thickening, microthrombi formation, and plexiform lesions.8
WHO PH CLASSIFICATION3,9
Identify which WHO group your PH patients are in.
I.PAH
II.PH associated with left heart disease
III.PH associated with lung disease and/or hypoxia
IV.ICTEPH
V.PH with unclear and/or multifactorial mechanism
Adempas is approved for adults with PAH (WHO Group 1) and inoperable or persistent/recurrent CTEPH (WHO Group 4) after surgery. Adempas has been studied predominantly in WHO functional class II-III patients.1
PAH=pulmonary arterial hypertension; PH=pulmonary hypertension.
- Adempas Prescribing Information. Whippany, NJ. Bayer Pharmaceuticals Inc., 2021.
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):180913.
- Humbert M, Kovacs G, Hoeper M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;00:1-114.
- Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S85-96.
- Freed DH, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(2):383-387.
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D92–D99.
- Condliffe R, Kiely DG, Gibbs SR, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177(10):1122-1127.
- Piazza G, Goldhaber SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2011;364(4):351-360.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–D41.